Welcome to another episode of Heat Treat Radio, a periodic podcast where Heat Treat Radio host, Doug Glenn, discusses cutting-edge topics with industry-leading personalities. Below, you can either listen to the podcast by clicking on the audio play button, or you can read an edited version of the transcript. To see a complete list of other Heat Treat Radio episodes, click here.
Audio: A Discussion with David Wolff, Nel Hydrogen, Part 1
In this conversation, Heat Treat Radio host, Doug Glenn, engages Nel Hydrogen Heat Treat Manager David Wolff in a conversation about hydrogen generation and its purposes. Find out more about what hydrogen is best used for, what hydrogen can do for your company, why hydrogen is preferred to nitrogen, and how to safely use it to the best effect.
Click the play button below to listen.
Transcript: A Discussion with David Wolff, Nel Hydrogen, Part 1
The following transcript has been edited for your reading enjoyment.
Doug Glenn (DG): We're here today with David Wolff from Nel Hydrogen and we're going to be talking a bit about on-site hydrogen generation. This really has come about because of an eBook that David and one of his colleagues, a gentleman by the name of Chris Van Name, and Heat Treat Today worked on together. The eBook was based on a presentation that you gave at FNA 2018.
Dave Wolff (DW): You're correct. The eBook was based on the FNA (Furnaces North America). I did an expansion on it for Fabtech 2019.
DG: I want our readers to know you before we jump into the content of the book. If you don't mind, Dave, would you just give us your name, rank, serial number, etc.

DW: I've been in the industrial gas industry for my whole career, (hard to believe), going well over 40 years now. I've been a little over 20 years at Nel Hydrogen. Before we were called Nel, we were called Proton Onsite. I joined relatively early in Proton's history. Proton was begun in order to commercialize attractively cost on-site hydrogen using water electrolysis. I found that incredibly exciting, as I came from the industrial gas industry, and I witnessed first hand the importance of having cost effective access to hydrogen in order to succeed in materials processing. Prior to Proton, I was with Messer, who is now back in the United States; and I was with Air Products for about 13 years prior to my time with Messer.
DG: So you've spent, let's say, 40 years in the industrial gases industry and most recently, and a good bulk of that time, with what was called Proton Onsite, now called Nel Hydrogen. For our reader's sake, Nel in the US is headquartered out of New England?
DW: Yes. Nel, in the US is headquartered in Wallingford, Connecticut, which was where Proton was based. Nel's worldwide corporate headquarters is in Norway. Nel is a corporation related to the historical Norsk Hydro, which has been around since 1927 and involved with water electrolysis since the early 20's.
DG: So today we want to talk about hydrogen, but we're going to talk specifically about on-site hydrogen generation. But before
we get there, if you don't mind Dave, give us a quick rundown on just the role of hydrogen in your normal, typical heat treat process. What does hydrogen do for us?
DW: You start with the fact that hydrogen is a reducing gas, which means that it can prevent or even reverse oxidation. For example, you can put oxidized parts through a hydrogen atmosphere furnace and they'll come out the other end, say if it's a belt furnace, bright and shiny. At the elevated temperatures used in metal thermal processing (heat treating), the rate of oxidation is increased, so you have to protect the metal so that it doesn't discolor from oxidation. And more concerning, oxidation will interfere with braze material flow in brazing and will prevent proper sintering of powder metal fabricated parts, so oxidation is a real problem in thermal processing.
DG: Right. So the reason of the brazing and whatnot is because of contamination on the surfaces, right? You don't get a solid braze or a solid sinter.
DW: Exactly. Now hydrogen is not the only reducing gas. CO (carbon monoxide) can also be used. But CO is highly toxic, so it is not routinely used, except if it's created incidentally in the process of making endo or exo gas.
Some people wonder why nitrogen alone is not sufficient as a heat treating atmosphere. It's inert, right? But it's essentially impossible to flow enough nitrogen through an atmosphere furnace to eliminate all of the oxygen molecules. And if you did try to flow that much nitrogen through the furnace, you would rob all of the heat out of the furnace. So the attractiveness about hydrogen is it grabs and immobilizes the stray oxygen molecules preventing oxidation but still enables you to manage the flow rate in your furnace.
DG: There are some vacuum furnace heat treaters who place a piece of metal or some substance inside of their furnace (they call it a 'getter'), which basically attracts those undesirable elements out of the atmosphere. In a sense, hydrogen (not exactly, but in a sense) can be kind of that 'getter' that goes and 'gets,' if you will, the oxygen pulls it out of that atmosphere, where nitrogen you have to be pushing it out. You'd have to be putting so much nitrogen through, you still might not get rid of all of the oxygen, whereas if you have some hydrogen, it pulls it out.
DW: You're exactly right. The hydrogen acts as a chemical 'getter' and so it's analogous. A couple of other things I should mention. In addition to its role as a reducing gas to prevent or reverse oxidation, hydrogen has the highest heat conductivity of any gas. So the high heat conductivity of hydrogen means that parts heat up faster in a hydrogen containing atmosphere, and they cool off faster too. The high heat conductivity allows for higher productivity by faster cycles in batch heat treating and faster transport speed through continuous furnaces likes belts and pushers. Parts heat up fast and they cool down quickly. The alternative, if you have lower hydrogen content in your atmospheres, is longer furnaces, slower belt speeds, or longer back furnace cycles.
DG: Coefficient heat transfer hydrogen is the best for pulling heat out or putting heat in, so you're looking at process efficiencies there as well.
DW: Productivity. One final thing. While vacuum furnaces are widely used and yield terrific results, a vacuum furnace creates an inert atmosphere, not a reducing atmosphere. So a high vacuum furnace can prevent oxidation, but typically not reverse it. So in many cases, a wisp of hydrogen is often used to create a partial pressure hydrogen atmosphere in vacuum furnaces. For example, for powder metallurgy, you enhance the sintering by reducing the surface oxidation on the powder particles.
DG: We've hit on what hydrogen can do, and I think we've already hit on this next question, which is the typical heat treat processes. Brazing you've mentioned, sintering you've mentioned; what else would we typically use a hydrogen atmosphere for?
DW: Let's start with making sure that people are aware that hydrogen is used only in furnaces which are designed for hydrogen
atmosphere. They have to have the right flow path, they have to have electrical parts and safety systems such as flame curtains, which are expressly designed to safely use hydrogen. Also, and importantly, the newest thermal processing equipment is highly automated for safe use of hydrogen. While hydrogen can be used safely in older equipment that is also designed to use hydrogen, it's important to follow procedures which are specifically designed around hydrogen use. So those are key considerations.
DG: I think we ought to emphasize the caveat that you're issuing. Hydrogen does have its issues, and we need to be careful with the use of hydrogen. So don't just go throw hydrogen into your furnace. It is very, very important that the safety concerns be followed.
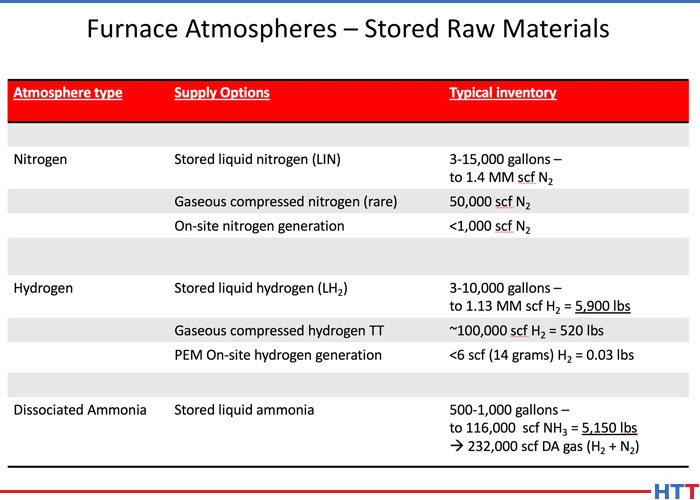
DW: So hydrogen is used to provide atmospheres for processes like annealing, brazing, glass metal sealing and all types of sintering including PM, MIM, and AM. Hydrogen is also widely used for processing magnetic materials, motor laminations and things like that. Keep in mind that both synthetic or blended atmospheres and also generated -- and by "generated" we typically refer to exo, endo and DA (dissociated ammonia) -- those atmospheres contain hydrogen as the primary reducing gas. As I mentioned earlier, exo and endo gas also contain CO, which is also a reducing gas, and exo and endo are often used in atmospheres for hardening. Typically you don't use a pure hydrogen atmosphere for that because that will tend to soften your parts.
DG: We've covered some of the processes that are involved, and you've alluded to this Dave, but let's flesh this out a little bit
more--we don't often use hydrogen alone. Often it is used as one component with other gases. Let's talk about why that is. Besides the obvious safety issues of using 100% hydrogen, let's talk about why we don't see 100% hydrogen and what we're often mixing with.
DW: I like to use an analogy here. Think of hydrogen gas in a furnace atmosphere, kind of like dish washing detergent. When you're washing dishes or processing parts, the function is to clean the parts, either the metal parts or cups and saucers. Dish washing detergent is diluted with water. Hydrogen is typically diluted with nitrogen or possibly with argon. In both cases, whether you're washing dishes or processing metal parts, the detergent is more expensive than the diluent. Hence, the idea is to use only as much detergent (hydrogen) as is needed to get the job done.
There are major differences between thermal processing and washing dishes. One major consideration is that the metal that is being thermally processed is actually chemically and metallurgically interacting with the furnace atmosphere. So you have the surface effect, which is the chemical effect, but also you have a metallurgical effect. That's how metals are softened and also, in the case of carbon, hardened. Obviously dishes are unaffected by the dish washing process other than having their surface cleaned. So that is part of the reason that atmosphere composition is greatly dependent on the metallurgy of the parts that you're processing. That is also the area where metallurgists have the greatest knowledge and provide unique process knowledge and value.
DG: So basically, you're going to use as little, if you will, or an appropriate portion of hydrogen to get the job done, and that is very much dependent on materials being run, processes being performed, etc. Correct?
DW: Exactly. The workhorse thermal processing atmosphere is a nitrogen atmosphere with a variable amount of hydrogen depending on the metal being processed. Carbon steel, for example, can be processed in a 4–5% hydrogen blend with the balance of the atmosphere being 95–96% nitrogen. This blend is so widely used that it has been given a nickname, so called forming gas. Some metals react adversely with hydrogen and cannot be processed in a hydrogen containing atmosphere at all. An example of that would be titanium. Titanium, which is so widely used for aerospace and also medical applications, is not processed in hydrogen at all, and that is why batch vacuum heat treating is so popular in aerospace and medical because there is a lot of titanium use.
DG: My understanding is that hydrogen causes embrittlement when we're dealing with titanium.
DW: Exactly. It causes damage to titanium parts. Batch processing also enables you to do lot tracking and other things which are important in both aerospace and medical.
Aluminum is another commonly heat treated metal that doesn't require hydrogen. Aluminum is basically generally heat treated in pure nitrogen. But other metals that do use hydrogen containing atmosphere include copper and brass, as I mentioned, magnetic steels and stainless steels. Generally, the steels, other than carbon steel, will require an atmosphere in the 30–60% range of hydrogen in nitrogen while certain grades of stainless must be heat treated in 100% hydrogen. Often the 300 series of stainless, people prefer to use 100% hydrogen for that.

End of Part 1.
Part 2 is scheduled to be released on February 13th. Check back here for a link to that episode or go to www.heattreattoday.com/radio after February 13, 2020, and look for Part 2 in the list of Heat Treat Radio episodes listed.








