![]() Your parts need heat treated to herculean surface hardness but with a soft, ductile core. That is to say, you are looking at case hardening processes, most likely one of these: gas carburizing, low-pressure carburizing, carbonitriding, gas nitriding, and ferritic nitrocarburizing.
Your parts need heat treated to herculean surface hardness but with a soft, ductile core. That is to say, you are looking at case hardening processes, most likely one of these: gas carburizing, low-pressure carburizing, carbonitriding, gas nitriding, and ferritic nitrocarburizing.
Mike Harrison at Gasbarre Thermal Processing Systems brings us a Technical Tuesday article about what case hardening is and how five of the most common processes vary by (1) comparing the specific guidelines for each temp and time, (2) identifying equipment used to perform each process, and (3) providing a chart (at the end!) to understand different process considerations.

Engineering Manager of Industrial Furnace Systems Division
Gasbarre Thermal Processing Systems
Case hardening falls into a class of heat treatment processes that typically involve the addition of carbon and/or nitrogen to the material through solid-gas reactions at the surface followed by diffusion. These processes are performed for any number of reasons that generally include increasing strength and wear resistance, but in all cases the end result is a harder, higher-strength surface with a softer, more ductile core.
Case hardening processes can be divided into two subsets: those that include quenching to harden, such as gas carburizing, low-pressure carburizing (LPC), and carbonitriding; and those that do not include quenching, such as gas nitriding and ferritic nitrocarburizing (FNC). This article will provide a brief look into each process, the types of equipment used, and considerations for implementation.
Diffusion + Quenching Processes
These processes involve heating the workload to austenitizing temperature, which is above the upper critical temperature for the material in question, then supplying and allowing the desired element(s) to diffuse into the part surface, followed by rapid cooling (quenching) to create a phase change to martensite that strengthens the material. Tempering is then performed to create a material that has the desired final strength and ductility properties. The result is a high concentration of added elements on the surface that continually decreases through diffusion until eventually matching the same concentration as the base material; this gradient similarly produces a hardness that is higher at the surface, gradually diminishing until reaching the core. Higher alloyed steels may also see a microstructural change in the core from quenching that produces a core with higher hardness than the previously untreated material, but lower than the surface hardness produced.
Atmosphere Gas Carburizing
Gas carburizing is a process where carbon is added to the material’s surface. The process is typically performed between 1550-1750°F, with carburizing times commonly between 2-8 hours; of course, these values can vary depending on the material, process, and equipment. The most common atmosphere used for atmosphere gas carburizing is endothermic gas with additions of either natural gas or propane to increase the carbon potential of the furnace atmosphere. Common case depths achieved are around 0.005-0.040”, with deeper cases possible through a combination of longer treatment times and/or higher temperatures.

The atmosphere gas carburizing process can be performed both in batch and continuous equipment. On the batch side, traditionally an integral quench (IQ) furnace is used (Fig. 1); it consists of a heating chamber where the workload is heated and exposed to the carburizing atmosphere, then the workload is transferred to an attached quench tank for cooling. The entire furnace system is sealed and under protective atmosphere to preserve the part surface and maintain safe control of any combustible gases. For batches of large product, a pit furnace can be used for carburizing with the workload being transferred via an overhead crane into and out of the furnace to a quench tank.
For continuous processing, a belt furnace can be used. The product is placed on a belt and then progresses through the furnace at the desired temperature and atmosphere composition; the carburizing time can be varied by adjusting the belt speed through the furnace. At the end of the furnace, the parts drop off the belt into the quench tank. Then, a conveyor pulls the parts out of the tank and drops them on another belt to be washed and tempered. For continuous processing of heavier loads pusher furnaces, rotary retort, rotary hearth, and roller hearth furnaces can be used.

To achieve a carburizing atmosphere endothermic gas is typically used, which is produced by an endothermic gas generator (Fig. 2) that heats a combination of natural gas and air to create a mixture that is approximately 40% hydrogen, 40% nitrogen, and 20% carbon monoxide. This mixture is generally considered carbon-neutral, meaning it will neither add nor deplete carbon from the surface. To increase the carbon concentration the endothermic gas needs to be enriched with a gas (typically natural gas or propane) that will help produce additional carbon monoxide, which will “boost” the carbon potential and drive carbon diffusion into the material.
A less common carburizing atmosphere comes from a nitrogen-methanol system, where nitrogen gas and liquid methanol are combined and injected into the furnace. Upon exposure to the high furnace temperature the methanol will decompose to hydrogen and carbon monoxide. Natural gas or propane additions are still required in order to provide carbon for absorption into the surface of the steel.
Low-Pressure Carburizing
Low-pressure carburizing (LPC), or vacuum carburizing, is a variation of carburizing performed in a vacuum furnace. Instead of the atmospheres mentioned previously, a partial pressure of hydrocarbon gas (such as propane or acetylene) is used that directly dissociates at the part surface to provide carbon for diffusion. After LPC, the workload is transferred to a quench system that could use oil or high-pressure gas, typically nitrogen. LPC with gas quenching can be an attractive option for distortion prone complex geometries as the cooling rates are slower than oil quenching; however, given the slower cooling rate, it becomes very important to choose a higher alloyed steel that will achieve the desired hardness.

LPC typically provides faster carburizing times when compared to traditional gas carburizing. This can be attributed to a more efficient reaction of the hydrocarbon gas used and to the option of using higher carburizing temperatures, typically up to 1900°F. This is made possible by the type of internal furnace construction of vacuum furnace design, although care must be taken at higher temperatures to avoid undesirable grain growth in the material. LPC also has the benefit of eliminating the potential for intergranular oxidation, since it is running in a vacuum system.
LPC is typically performed in a single-chamber vacuum furnace, with oil quenching or high-pressure gas quenching done in a separate chamber (Fig. 3). Continuous vacuum furnaces can also be used in applications that require increased throughput (Fig. 4).

Carbonitriding
Despite its name, carbonitriding is more closely related to carburizing than it is to nitriding. Carbonitriding is a process where both carbon and nitrogen are added to the material surface. This process is typically performed in a range of 1450-1600°F and generally produces a shallower case depth than carburizing. Carbonitriding is used instead of carburizing for plain carbon steels that do not contain enough alloying content to respond well to quenching, as the added nitrogen can provide a higher hardenability in the case to allow for proper hardness development.
Atmosphere carbonitriding can be performed in the same equipment as is used for carburizing. The furnace atmosphere is still typically endothermic gas-based and includes the addition of ammonia to provide the nitrogen. Vacuum carbonitriding with both hydrocarbon and ammonia additions can also be performed in the same equipment as used for vacuum hardening and low pressure carburizing.
Diffusion Only Processes
These processes involve heating the workload to a temperature below the austenitizing temperature, allowing the desired element(s) to diffuse into the part surface, then slow cooling. The increase in hardness at the material surface comes only from the addition of the diffused element(s), and not from a phase change due to quenching. As these processes are performed below the lower critical temperature (i.e., below the austenitizing range), the desired core hardness and microstructure need to be developed through a separate heat treatment prior to case hardening. Generally, the process temperature selected should be at least 50°F below any prior treatment temperatures to avoid impact to the core properties.
Gas Nitriding
Gas nitriding is a process where nitrogen is added to the material surface. The process is typically performed between 925-1050°F; cycle times can be quite long as the diffusion of the nitrogen is slow at these temperatures, with nitriding times typically ranging from 16 - 96 hours or more depending on the material and case depth required. Nitriding can be performed in either a single or two-stage process and has the potential to produce two types of case, the first being a nitrogen-rich compound layer (or “white layer”) at the surface that is extremely hard and wear-resistant but also very brittle. This compound layer depth is dependent on processing time. In the more traditional two-stage process, the case depth produces a gradient of hardness from surface to core that commonly ranges from 0.010-0.025”, with minimal white layer, typically between 0-0.0005”. Nitriding is typically performed on higher alloyed steels or steels specifically designed for the nitriding process (e.g., Nitralloy®) as it relies on the formation of nitrides to create the increased hardness, which is achieved through the use of nitride-forming alloys such as aluminum, molybdenum and chromium. Pre and post oxidation treatments can be incorporated into the cycle to achieve certain benefits. Since the process does not require quenching to harden, it has the potential of producing a product that is more dimensionally stable and may not require any post-process finishing.

This process is most commonly performed in batch equipment; while it is possible to use a continuous furnace, keeping the ends of furnace sealed to contain the atmosphere can be challenging. Traditionally, pit furnaces have been used for nitriding as they can accommodate larger load sizes and can be easier to seal as gravity helps keep the lid sealed; however, horizontal designs have gained in popularity in recent years (Fig. 5). In either case, the furnaces are usually a single-chamber design with the load sealed inside an Inconel or stainless steel retort.
To achieve a nitriding atmosphere, ammonia (not nitrogen) is used to supply the atomic nitrogen necessary for diffusion. At the process temperatures used, ammonia does not readily dissociate on its own; rather, it dissociates when exposed to a heated steel surface (iron acting as a catalyst) into atomic nitrogen and hydrogen. To control the amount of nitrogen available for nitriding, the dissociation rate of the ammonia can be measured with high dissociation rates (high hydrogen content) providing a lower nitriding potential and low dissociation rates (low hydrogen content) leading to more nitriding potential. The depth of the compound layer can be varied through control of the nitriding potential, with higher nitriding potentials producing a thicker compound layer.
For more precise atmosphere control, an ammonia dissociator can be used to provide gas to the furnace that has already been split to dilute the atmosphere with hydrogen to more quickly achieve a high dissociation rate in the furnace. The ammonia dissociator is a heated box with a small retort inside; the ammonia is passed through this retort that contains a catalyst to promote the dissociation of the ammonia, and the resulting gas mixture is cooled and then injected into the furnace.
Ferritic Nitrocarburizing
In the author’s opinion, just like with carbonitriding, ferritic nitrocarburizing (FNC) is named incorrectly as it is more closely related to nitriding than it is with carburizing. FNC is a process that is still mostly nitrogen-based but with a slight carbon addition as well. The added carbon helps promote compound layer formation, particularly in plain carbon and low alloy steels that do not contain significant nitride-forming alloys. This process is typically performed in a range of 1025-1125°F with cycle times much shorter than nitriding, typically 1-4 hours. The compound layer produced is usually much deeper than nitriding at 0.0005-0.0012”, with case depths reaching up to 0.025”, although in many applications a case depth may be difficult to measure. FNC is usually performed instead of nitriding in applications where the deeper compound layer is needed to increase wear resistance, but the added strength of a deep case depth is not as critical.
FNC can be performed in the same equipment used for nitriding, as long as a hydrocarbon gas is available to the furnace such as carbon dioxide or endothermic gas. FNC can also be performed in an IQ furnace using a mixture of ammonia and endothermic gas; for cooling, the parts can be oil quenched or slow cooled in a top cool chamber (if equipped).
Considerations
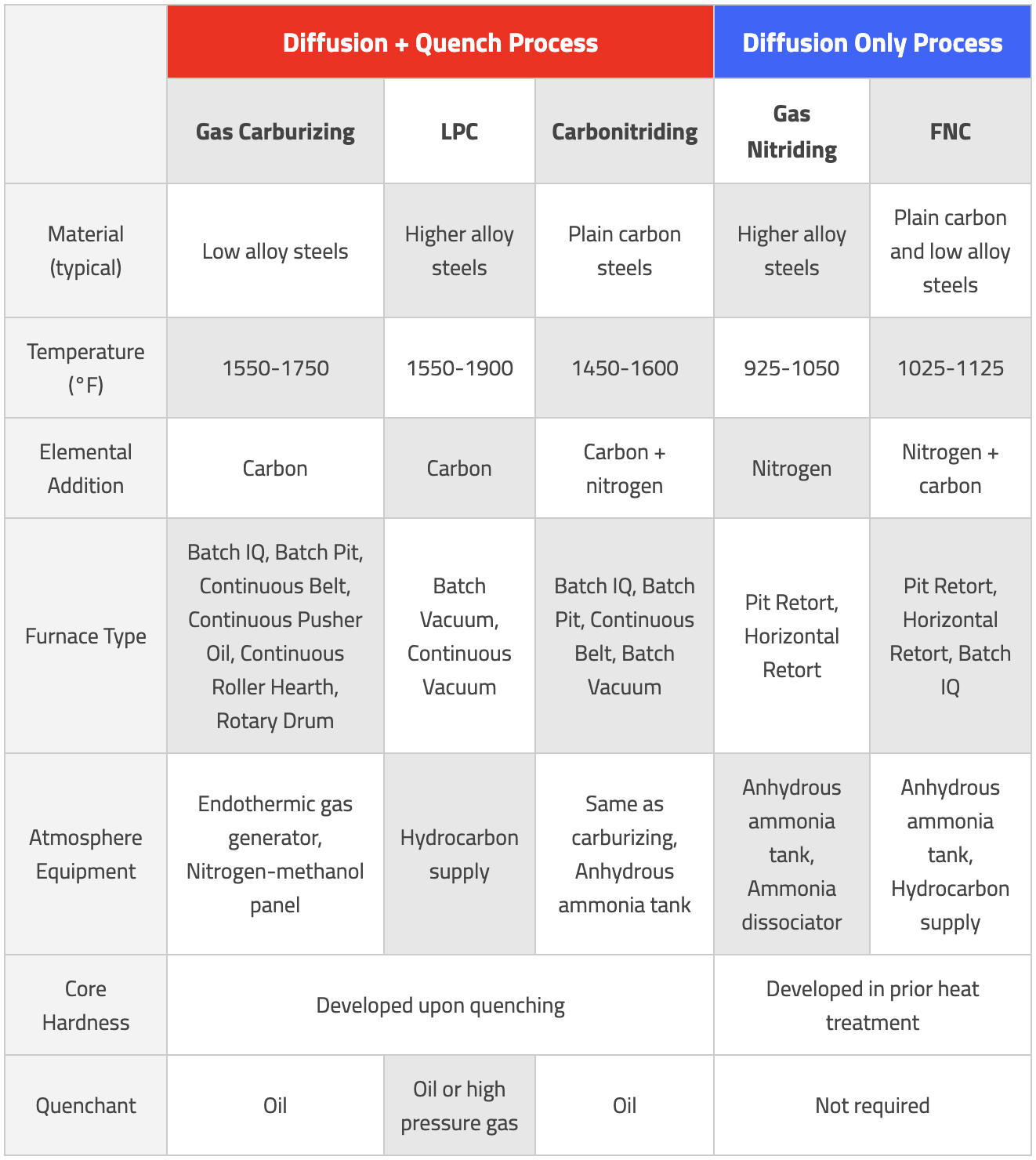
Case hardening processes are some of the most common heat treatments performed, but each process has its own unique needs. The table below provides a summary of the considerations that need to be made when selecting the optimum process. This list is by no means exhaustive; it is encouraged to work with a furnace manufacturer familiar with each process to help select the correct process and equipment needed.
About the Author: Mike Harrison is the engineering manager of the Industrial Furnace Systems division at Gasbarre. Mike has a materials science and engineering degree from the University of Michigan and received his M.B.A. from Walsh College. Prior to joining Gasbarre, Mike had roles in metallurgy, quality, and management at both captive and commercial heat treat facilities, gaining nearly 20 years of experience in the thermal processing industry. Gasbarre provides thermal processing equipment solutions for both atmosphere and vacuum furnace applications, as well as associated auxiliary equipment and aftermarket parts & service.
For more information: Contact Mike at mharrison@gasbarre.com