![]() What are the factors that lead to carburization and carbon transmission? How can heat treater avoid these unwanted reactions? Discover the challenges of CFC fixtures and the steps heat treaters can take to mitigate these challenges.
What are the factors that lead to carburization and carbon transmission? How can heat treater avoid these unwanted reactions? Discover the challenges of CFC fixtures and the steps heat treaters can take to mitigate these challenges.
This Technical Tuesday article, written by Dr. Jorg Demmel, founder, 0wner, and president, High Temperature Concept, was first published in Heat Treat Today's March 2023 Aerospace Heat Treating print edition.
Introduction

Founder, Owner, President
High Temperature Concept
The main advantages of CFC fixtures were introduced in “CFC Fixture Advantages and Challenges in Vacuum Heat Treatment, Part 1,” which was released in Heat Treat Today’s November 2022 publication. This included a discussion of the limits of CFC in vacuum and protective atmosphere heat treatment. Successful applications of CFC workpiece carriers in heat treatment were presented along with field test results that included a brief discussion of undesired contact reactions (i.e., carburization and melting of parts). In Part 2 of this paper, the mechanisms involved with carburization and carbon transmission due to direct contact of parts with CFC fixtures will be further explained.
Mass Transfer from CFC Fixtures

The mass transport of carbon from CFC fixtures into steel parts at high temperatures will be examined in the following areas:
- Reactions in oxygen (i.e., the reaction medium)
- Transport of carbon in CFC during exposure to oxygen
- Transfer mechanism into the steel parts
- Diffusion of carbon into the steel parts
- Part reactions (melting, carbide formation)

Source: Dr. Jorg Demmel, High Temperature Concept
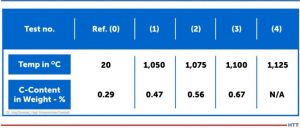
CFC samples were tested in contact with steel samples under laboratory conditions in a vacuum of 7.5 x 10-7 Torr (1 x 10-6mbar). Results of the contact with CFC for steel samples at different temperatures are presented to the left (Figure 1). It is important to note that:
- Sample (0) is the reference sample and had no exposure to the contact test.
- Sample (0’) is the back side of Sample (0).
- Sample (1) is the contact side at 1922°F (1050°C).
All three samples are visually identical, therefore only one is shown. Sample (2) at 1967°F (1075°C) and Sample (3) at 2012°F (1100°C) exhibited a distinct visual surface pattern after CFC contact. This was analyzed by Glow Discharge Optical Emission Spectroscopy (GDOES) and the test location (gray spot) clearly observed on Samples (2) and (3). For Sample (4) run at 2057°F (1125°C), the CFC was found to have adhered to the steel surface.
The carbon content in 10mm depth measured with GDOES (see the profiles in Figure 1) increased from initially 0.29 weight-% for the 1922°F (1050°C) test, although nothing was visible on metal surfaces. For carbon contents, see Table 1.
Source: Dr. Jorg Demmel, High Temperature Concept
CFC Reactions with Oxygen
The chemical reactions of CFC with various gases are essential in Step 1 (referenced in Part 1 of this article) and an indicator of chemical thermal suitability.
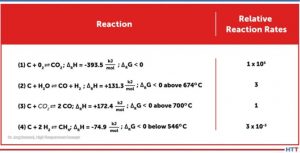
In the case of the unwanted contact carburization considered above is similar, in a sense, to carburization of steel in contact with carbon powder or granulate. However, the actual carburization mechanism, which occurs between approximately 1616°F and 1697°F (880°C and 925°C), does not take place directly via the carbon contact but is based on the fact that solid carbon reacts with atmospheric oxygen according to the Equation Table to form carbon dioxide (CO2).
Source: Dr. Jorg Demmel, High Temperature Concept
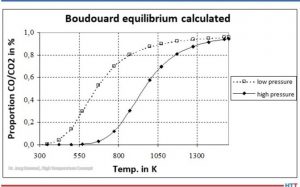
Carbon monoxide (CO) is then formed from CO2 by the Boudouard reaction (Equation 3). At high temperatures and low pressures (see Figure 2), almost only CO is present.
Source: Dr. Jorg Demmel, High Temperature Concept
Transport of Carbon
The carbon carrier must be transported to the surface of the parts.
The cases considered in Part 1 of this article were conducted in vacuum, that is in the absence of a carburizing atmosphere. The laboratory tests were even carried out in a vacuum as low as 7.5 x 10-7 Torr (1 x 10-6mbar). Nevertheless, part surface reactions were observed.
Transfer Mechanism into the Steel Parts
Theoretically, carbon from the CFC fixtures can be transferred into the steel via solid phase (as opposed to gaseous phase) reactions. Gas particles can be adsorbed by surfaces via physisorption and/or chemisorption. The author’s personal research experience has shown that metal samples usually oxidize after a short time, even in a high vacuum of 7.5 x 10-7 Torr (1 x 10-6mbar). In particular, elements such as iron, molybdenum, and chromium have a strong ability to chemically adsorb oxygen or CO.
Furthermore, there is a disproportionately large amount of adsorbed oxygen in the CFC samples. CFC has open porosities as high as 30%. CFC in industrial practice is never completely evacuated. So, there is a disproportionately large amount of oxygen present in CFC fixtures.
It can be assumed that oxygen repeatedly escapes from the CFC and is initially available in the contact area. Proof of this can be provided by the GDOES analysis. Outside the contact areas, no (gas) carburization took place (as evidenced by the non-contact side of steel samples).
The oxygen and carbon surplus combined with close contact lead to complete reaction of oxygen creating carbon dioxide as in Equation (1). Because of the carbon surplus, almost only carbon monoxide is produced as shown in Equation (2). Because of the very close contact between CFC and steel, C-adsorption by gamma iron and desorption of carbon dioxide as in Equation (5) takes place:

Source: Dr. Jorg Demmel, High Temperature Concept
Since carbon dioxide immediately comes in contact with carbon in the CFC again, carbon monoxide is produced according to Equation (3). In other words, carbon dioxide regenerates immediately and the reaction starts again.
Direct carbon transfer from CFC to metal via solid phase is very unlikely since carbon atoms in CFC are firmly bound in rings.
Diffusion of Carbon in the Steel Parts
In solids, the surface diffusion usually takes place at significantly higher diffusion rates than in the bulk material. The thermodynamic driving force of diffusion or carburizing reactions is the difference in carbon activity for a specific concentration in the austenite to that of the reaction medium. The carbon activity is the ratio of the vapor pressure of the carbon in state under consideration to vapor pressure of pure carbon (graphite/CFC). Alloying elements of the steel influence the activity of the carbon.
Part Reactions (Melting and Carbide Formation)
Steel can begin to melt if, at the given values for temperature and pressure, a partially liquid phase is reached, that is, the solidus line in the phase diagram is exceeded. At even higher temperatures, the liquidus temperature can be reached and steel is completely liquid.
According to metastable iron-carbon diagram phase diagram (Figure 3), a steel such as SAE/ AISI 4340 (34CrNiMo6) alloy (DIN 1.6582) with around 0.47% by weight percent carbon does not begin to melt at 1922°F (1050°C), the exposure temperature for Sample (1), or Sample (2) at 0.56% and 1967°F (1050°C) for Sample (3) with 0.67% for 2012°F (1100°C). The iron-iron carbide phase diagram applies to steels with less than 5% (by mass) of alloying elements and thermodynamic equilibrium, so it is an accurate representation for a SAE/AISI 4340 (34CrNiMo6) alloy.
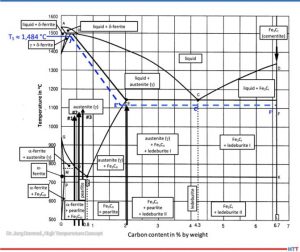
Source: Dr. Jorg Demmel, High Temperature Concept
A calculation of the solidus temperature shown on the iron-iron carbide diagram (Figure 3), which is dependent on the carbon content and alloying elements, yields a value of 2703.2°F (1,484°C) (J’).
For an SAE/AISI 4340 (34CrNiMo6) steel (DIN 1.6582) with 0.3% C and one for 0.5% C, the calculated solidus temperature is 2640°F (1449°C). This is shown on the J’-E’ blue dotted line in Figure 3. In other words, a lower solidus line (cf. dashed blue line in Figure 3) and thus a slight reduction in austenite phase region.
The iron-carbon diagram also indicates that melting of surfaces that have absorbed carbon (e.g., Sample No. 2) will occur at 1967°F (1075°C). This value is within approximately 90°F (50°C) of the temperature used (dotted line E’-C’-F’). From this information we can conclude that the observations seen in Figure 1 are not the result of melting, but rather imprints due to surface softening.
The melting (c.f., Figure 1) observed in Test No. 4, which occurred at 2057°F (1125°C) is likely due to partial carburization of the steel surface and exceeding the solidus temperature. A micrograph confirms eutectic melting and high carbon content, which could also be indirectly confirmed by hardness measurement.
Carbide Formation
Additional reactions can occur between carbon absorbed from the CFC fixtures and the steel parts due to either separation of carbides (e.g., iron carbide in the form of secondary cementite) or carbide formation with alloying elements such as Ti, V, Mo, W, Cr, or Mn (listed in decreasing tendency to form carbides).

Source: Dr. Jorg Demmel, High Temperature Concept
Table 2 lists various elements in alphabetical order that react with carbon above the specified temperatures to form reaction products mentioned, primarily carbides. It should be noted that the temperatures listed apply only to pure metals and pure carbon. As such, they provide only rough approximations of a temperature at which a reaction might begin.
Countermeasures
There are several measures to avoid these unwanted reactions:
- Ceramic oxide coatings such as aluminum oxide (Al2O3) or zirconium oxide (ZrO2) layers placed onto the CFC
- Hybrid CFC fixtures having ceramics in key areas to avoid direct contact with metal workpieces
- Alumina composite sheets
- Boron nitride sprays
- Special fixtures made of oxide ceramics
An yttrium-stabilized zirconium oxide layer (93/7) was applied to CF222 by thermal plasma spray and tested successfully (see Figure 4).

Source: GTD Technologie Deutschland
Summary
It is important to consider the specific process conditions in advance so that unwanted reactions — from carburization to catastrophic melting of the workpieces — can be avoided. Effective countermeasures can be taken.
References
Atkins, P. W.: Physikalische Chemie. 1. vollst. durechges. u. berichtigter Nachdr.d. 1. Aufl ., Weinheim, VCHVerlag, 1988 – ISBN 3-527-25913-9.
Bürgel, R.: Handbuch Hochtemperatur-Werksto technik: Grundlagen, Werksto bean-spruchungen, Hochtemperaturlegierungen. Braunschweig, Wiesbaden: Vieweg, 1998. ISBN 3-528-03107-7.
Demmel, J.: Advanced CFC-Fixture Applications, their scientific challenges and economic benefits, In: 30th Heat Treating Society Conference & Exposition, Detroit, MI, USA, 15th Oct. 2019.
Demmel, J.: Werkstoffwissenschaftliche Aspekte der Entwicklung neuartiger Werkstückträger für Hochtemperaturprozesse aus Faserverbundkeramik C/C und weiteren Hochtemperaturwerkstoffen, Dissertation, TU Freiberg, Germany, 2003.
Demmel, J.: Why CFC-Fixtures are a Must for Modern Heat Treaters, FNA 2020 Technical Session Processes & Quality, USA, 30th Sept. 2020.
Demmel, J., et al: Applications of CMC-racks for high temperature processes. In: 4th Int. Conf. on High-Temperature Ceramic Matrix Composites, 3.10.2001, p. A-17.
Demmel, J. und J. Esch: Handhabungs-Roboter sorgt für Wettbewerbsvorsprung. Härterei: Symbiose von neuen Werkstoffen und Automatisierung. In: Produktion (1996), No. 16, p. 9.
Demmel, J. und U. Nägele: CFC revolutioniert die Wärmebehandlung. In: 53. Härterei-Kolloquium, Wiesbaden, 10.10.97. Vortrag und Tagungsbericht.
Demmel, J., Lallinger, H.: CFC-Werkstückträger revolutionieren die Wärmebehandlung. In: Härtereitechnische Mitteilungen 54, No. 5, p. 289-294, 1999.
Eckstein, H.-J., et al: Technologie der Wärmebehandlung von Stahl. 2nd Edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1987. ISBN 3-342-00220-4.
Godziemba-Maliszewski, J.; Batfalsky, P.: Herstellung von Keramik-Metall-Verbindungen mit Diffusionsschweißverfahren. In: Technische Keramik, Jahrbuch, Essen, 1 (1988), S. 162-172. ISBN 3-80272141-1.
Grosch, J.: Grundlagen-Verfahren-Anwendungen-Eigenschaften einsatzgehärteter Gefüge und Bauteile, ExpertVerlag, 1994, ISBN 3-8169-0739-3.
Hollemann, A.F.; Wiberg, E.: Lehrbuch der anorganischen Chemie / Hollemann-Wiberg. 91.-100. Aufl ., de Druyter Verlag, 1985 – ISBN 3-11-007511-3.
Kriegesmann, J.: Technische Keramische Werkstoffe. Loseblattwerk mit 6 Ergänzungslieferungen pro Jahr.
Kussmaul, K.: Werkstoffkunde II. Stuttgart, Universität, Lehrstuhl für Materialprüfung, Werkstoffkunde und Festigkeitslehre, Vorlesungsmanuskript, 1993.
Lay, L.: Corrosion Resistance of Technical Ceramics. 1. Aufl ., Teddington, Middlesex, Crown-Verlag, 1983 – ISBN 0-11-480051-0.
Marsh, H.; u.a.: Introduction to Carbon Science. 1. Aufl ., London, Butterworths-Verlag, 1989 – ISBN 0-40803837-3.
Spur, G.: Wärmebehandeln. Berlin, 1987, ISBN 3-446-14954-6.
Samsonow, G.V.: Handbook of refractory compounds. New York, 1980.
Schulten, R.: Untersuchungen zum Kohlenstofftransportmit Carbidbildung in Nickelbasis-legierungen. RWTH Aachen, Fakultät für Maschinenbau, Diss., 1988 Deutsche Keramische Gesellschaft, 1990 following. ISBN 3-87156-091-X.
About the Author: Dr. Jorg Demmel is the founder, owner, and president of High Temperature Concept. He received his Engineering Doctorate in the field of CFC workpiece carriers for heat treatment and served in different leading positions for Volkswagen before moving to the U.S. In this article, Demmel draws on his dissertation, “Material scientific aspects of the development of new Fixtures for high temperature processes made of fiber-composite ceramics C/C and other high temperature materials” (Technical University Mining Academy Freiberg, Germany, 2002/3), and his personal experiences. For more information, contact Jorg at jorg.demmel@high-temperature-concept.com
 Find heat treating products and services when you search on Heat Treat Buyers Guide.com
Find heat treating products and services when you search on Heat Treat Buyers Guide.com