![]() What happens when a lead engineer sticks his head in new advancements in materials from NASA? For the author of this article, it means the successful research and development of a new generation of workpiece carriers and fixtures made from “a high-tech ceramic matrix composite of very strong carbon fiber,” that is, CFC.
What happens when a lead engineer sticks his head in new advancements in materials from NASA? For the author of this article, it means the successful research and development of a new generation of workpiece carriers and fixtures made from “a high-tech ceramic matrix composite of very strong carbon fiber,” that is, CFC.
This Technical Tuesday article, written by Dr. Jorg Demmel, founder, 0wner, and President, High Temperature Concept, was first published in Heat Treat Today's November 2022 Vacuum print edition.
Introduction: From NASA to Industrial Heat Treatment

Founder, Owner, President
High Temperature Concept
In the mid-1990s, a development in materials from NASA moved into my focus. I was an associate and lead engineer at the Fraunhofer Institute in Stuttgart, Germany, so I posed the question: Could CFC material (carbon fiber-reinforced carbon) substitute for non-abrasion-resistant and brittle graphite as the material used for workpiece carriers in the soldering process of drills? The answer: yes. The story did not end here. This project, which included the automated handling of the drills in some continuous furnaces, was just the first accomplishment. What ensued was a successful research and development of a new generation of workpiece carriers and fixtures made from CFC (“Carbon Fiber Carbon”).
Material Properties and Main Advantages of CFC

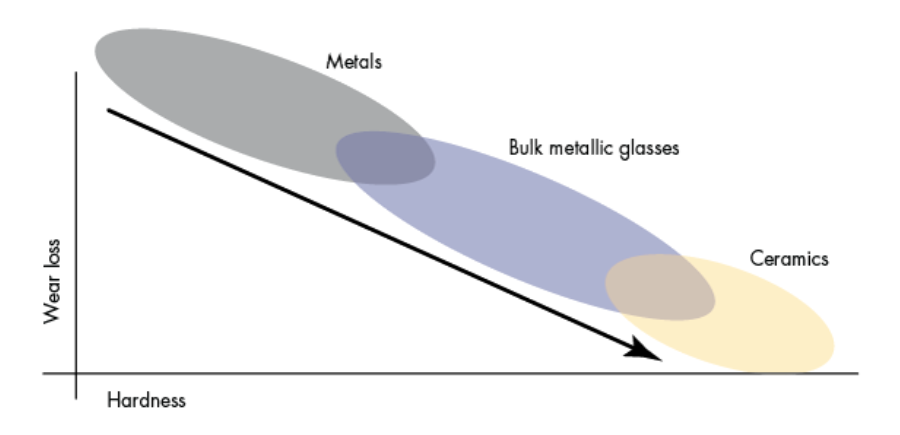
CFC (aka, CFRC, or C/C), which stands for carbon fiber-reinforced carbon, is a high-tech ceramic matrix composite of very strong carbon fibers (or fiber rovings) in a compensative carbon (graphite) matrix. Material properties of some relevant heat treatment fixture materials were evaluated, and some are shown in Figure 1. These CFC properties have the following positive effects when used as CFC fixtures for heat treatment:
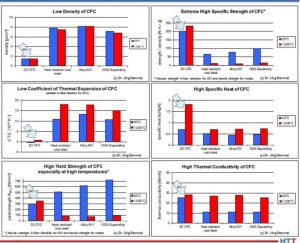
- Because of their low density, CFC fixtures have a lower weight than their steel alloy counterparts (about five times), which reduces the efforts for manual handling.
- Because of the increased strength of CFC at high temperature, the fixture weight can be reduced further. Additionally, fixture volume can be reduced — in some applications dramatically — so that, when combined with a specific CFC fixture design, furnace capacities can be increased up to 100%.
- The following characteristics of CFC fixtures are responsible for the longer fixture life cycles (up to greater than five times), less workpiece distortion and rework, and make an automatic workpiece handling possible for the first time ever: the low CTE (coeffcient of thermal expansion) value for CFC in the direction of the fiber, the fact that CFC is chemically inert in vacuum or
certain protective atmospheres, has an excellent thermal shock resistance, and it doesn’t grow, creep, or age like metals. - Although the specific heat of CFC is higher, the energy consumption can be reduced and shorter heating up and cooling down times can be reached, resulting in up to 30% shorter process cycle times for the same workpieces.

Figure 2 shows all potential advantages of CFC fixtures compared to state-of the- art steel alloy; a short payback time of the investment with high profitability are possible.
CFC Fixture Suitability in Vacuum Heat Treatment
Since CFC is made of carbon, it is not made for high temperatures above 752°F (400°C) in air or atmosphere with high percentages of oxygen, water vapor, hydrogen, or carbon dioxide for long periods of time. Therefore, vacuum or protective gas atmospheres are, in general, a suitable environment for CFC fixtures.

Table 1 shows the relative reaction rates for graphite according to H. Marsh in Introduction to Carbon Science, 1989 in the “reaction controlled” Zone I up to 1472°F (800°C) under oxygen, steam (H2O) Figure 3. Burning rates of graphite as a function of temperature
Industrial experience shows that CFC under vacuum of < 10-2 mbar at 1472°F or 1832°F (800°C or 1000°C) at a low dew point < -4°F (-20°C) (< 0.1 % vapor content) lasts at least 5,000 hours (real process time). At 3632°F (2000°C), the life is about 2,000 hours. Dew points of about 0°C (about 0.6 % vapor) cause higher reaction rates and reduce lifetime to about 800 to 1,000 hours.
Unwanted Contact Reactions
Contact reactions between the CFC fixtures and the workpieces, primarily made of steel, can lead to changes in the workpieces: for example, carburization of the workpiece in contact with the CFC. It is important to avoid these contact reactions since the properties of the workpieces must under no circumstances be changed in an uncontrolled manner. Neither the chemical composition nor mechanical properties nor the surface may change beyond the permissible tolerance limits. The CFC fixture should also not be subject to any changes that could adversely affect its properties and, above all, its service life.
The following materials, consisting of mainly workpiece materials made of steel, were used in direct contact with CFC, especially in heat treatment and brazing. CFC 1501G (SGL), CF222 (Schunk), or CX-27C1 (GTD, Toyo Tanso) were used as CFC workpiece carrier materials. Table 2 gives an overview of the results. The symptoms columns with “none” indicate no problems. The colored cells showed problems. The last column references the application or the results.
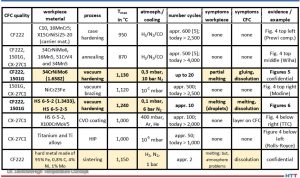


The contact partners and processes in which unwanted contact reactions occurred in the field test (colored in Table 2) and which are not confidential (bold font) are examined more closely in Part 2. See Figure 5 which shows some contact reactions on tempered steel drills after vacuum hardening at 2066°F (1130°C) under vacuum of 0.3 mbar (0.3 hPa or 225 mm Hg or “micron”).

Figure 6 shows some heavy melting reactions of high-speed steel after vacuum hardening at 2264°F (1240°C) under vacuum of 0.1 mbar (0.1 hPa or 75 mm Hg or “micron”).
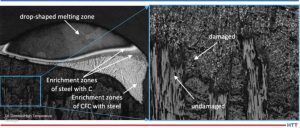
The carbon transmission mechanism with unwanted carburization, along with eutectic reaction of some workpieces made of steel with CFC, and some technical solutions will be explained in Part 2 of this article.
References
Atkins, P. W.: Physikalische Chemie. 1. vollst. durechges. u. berichtigter Nachdr.d. 1. Aufl., Weinheim, VCHVerlag, 1988 – ISBN 3-527-25913-9.
Bürgel, R.: Handbuch Hochtemperatur-Werkstofftechnik: Grundlagen, Werkstoffbean-spruchungen, Hochtemperaturlegierungen. Braunschweig, Wiesbaden: Vieweg, 1998. ISBN 3-528-03107-7.
Demmel, J.: Advanced CFC-Fixture Applications, their scientific challenges and economic benefits, In: 30th Heat Treating Society Conference & Exposition, Detroit, MI, USA, 15th Oct. 2019.
Demmel, J.: Werkstoffwissenschaftliche Aspekte der Entwicklung neuartiger Werkstückträger für Hochtemperaturprozesse aus Faserverbundkeramik C/C und weiteren Hochtemperaturwerkstoffen, Dissertation, TU Freiberg, Germany, 2003.
Demmel, J.: Why CFC-Fixtures are a Must for Modern Heat Treaters, FNA 2020 Technical Session Processes & Quality, USA, 30th Sept. 2020.
Demmel, J., et al: Applications of CMC-racks for high temperature processes. In: 4th Int. Conf. on High-Temperature Ceramic Matrix Composites, 3.10.2001, p. A-17.
Demmel, J. und J. Esch: Handhabungs-Roboter sorgt für Wettbewerbsvorsprung. Härterei: Symbiose von neuen Werkstoffen und Automatisierung. In: Produktion (1996), No. 16, p. 9.
Demmel, J. und U. Nägele: CFC revolutioniert die Wärmebehandlung. In: 53. Härterei-Kolloquium, Wiesbaden, 10.10.97. Vortrag und Tagungsbericht.
Demmel, J., Lallinger, H.: CFC-Werkstückträger revolutionieren die Wärmebehandlung. In: Härtereitechnische Mitteilungen 54, No. 5, p. 289-294, 1999.
Eckstein, H.-J., et al: Technologie der Wärmebehandlung von Stahl. 2nd Edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1987. ISBN 3-342-00220-4.
Godziemba-Maliszewski, J.; Batfalsky, P.: Herstellung von Keramik-Metall-Verbindungen mit Diffusionsschweißverfahren. In: Technische Keramik, Jahrbuch, Essen, 1 (1988), S. 162-172. ISBN 3-80272141-1.
Grosch, J.: Grundlagen-Verfahren-Anwendungen-Eigenschaften einsatzgehärteter Gefüge und Bauteile, ExpertVerlag, 1994, ISBN 3-8169-0739-3.
Hollemann, A.F.; Wiberg, E.: Lehrbuch der anorganischen Chemie / Hollemann-Wiberg. 91.-100. Aufl ., de Druyter Verlag, 1985 – ISBN 3-11-007511-3.
Kriegesmann, J.: Technische Keramische Werkstoffe. Loseblattwerk mit 6 Ergänzungslieferungen pro Jahr.
Kussmaul, K.: Werkstoffkunde II. Stuttgart, Universität, Lehrstuhl für Materialprüfung, Werkstoffkunde und Festigkeitslehre, Vorlesungsmanuskript, 1993.
Lay, L.: Corrosion Resistance of Technical Ceramics. 1. Aufl ., Teddington, Middlesex, Crown-Verlag, 1983 – ISBN 0-11-480051-0.
Marsh, H.; u.a.: Introduction to Carbon Science. 1. Aufl ., London, Butterworths-Verlag, 1989 – ISBN 0-40803837-3.
Spur, G.: Wärmebehandeln. Berlin, 1987, ISBN 3-446-14954-6.
Samsonow, G.V.: Handbook of refractory compounds. New York, 1980.
Schulten, R.: Untersuchungen zum Kohlenstofftransportmit Carbidbildung in Nickelbasis-legierungen. RWTH Aachen, Fakultät für Maschinenbau, Diss., 1988 Deutsche Keramische Gesellschaft, 1990 following. ISBN 3-87156-091-X.
About the Author: Dr. Jorg Demmel is the founder, owner, and president of High Temperature Concept. He received his Engineering Doctorate in the field of CFC workpiece carriers for heat treatment and served in different leading positions for Volkswagen before moving to the U.S. In this article, Demmel draws on his dissertation, “Material scientific aspects of the development of new Fixtures for high temperature processes made of fiber-composite ceramics C/C and other high temperature materials” (Technical University Mining Academy Freiberg, Germany, 2002/3), and his personal experiences. For more information Contact Jorg at jorg.demmel@high-temperature-concept.com
 Find heat treating products and services when you search on Heat Treat Buyers Guide.com
Find heat treating products and services when you search on Heat Treat Buyers Guide.com