Market Manager
Hubbard-Hall
“Successful heat treating begins by understanding the make-up of the steel that is to be treated.”
Heat Treat Today’s Technical Tuesday feature provides an overview of the heat treatment process and the benefits wrought from heat treating in salt baths. The article also illuminates details to understand part composition and the austempering and quenching process as a whole.
The author of this Original Content article, Jerry Dwyer, market manager at Hubbard-Hall, has previously written for Heat Treat Today on the topic of polymer quenchants as an alternative to water and oil quenching. Read more here.
Heat treating is a process in which metal is heated to a predetermined temperature and then cooled in a particular manner to alter its internal structure for obtaining a desired degree of physical, mechanical and metallurgical properties. The purpose is to obtain maximum strength (i.e., increase the metal’s hardness) and durability in the material.
Numerous industries utilize heat treated parts, including those in the automotive, aerospace, information technology, and heavy equipment sectors. Specifically, manufacturers of items such as saws, axes, cutting tools, bearings, gears, axles, fasteners, camshafts, and crankshafts all rely on heat treating to make their products more durable and to last longer.1
The heat treating processes require three basic steps:
- Heating to a specified temperature.
- Holding at that temperature for the appropriate amount of time.
- Cooling according to prescribed methods.
Understanding the Part Material
According to the ASM International’s Heat Treating Society, about 80 percent of heat treated parts are made of steel, such as bars and tubes, as well as parts that have been cast, forged, welded, machined, rolled, stamped, drawn, or extruded.1
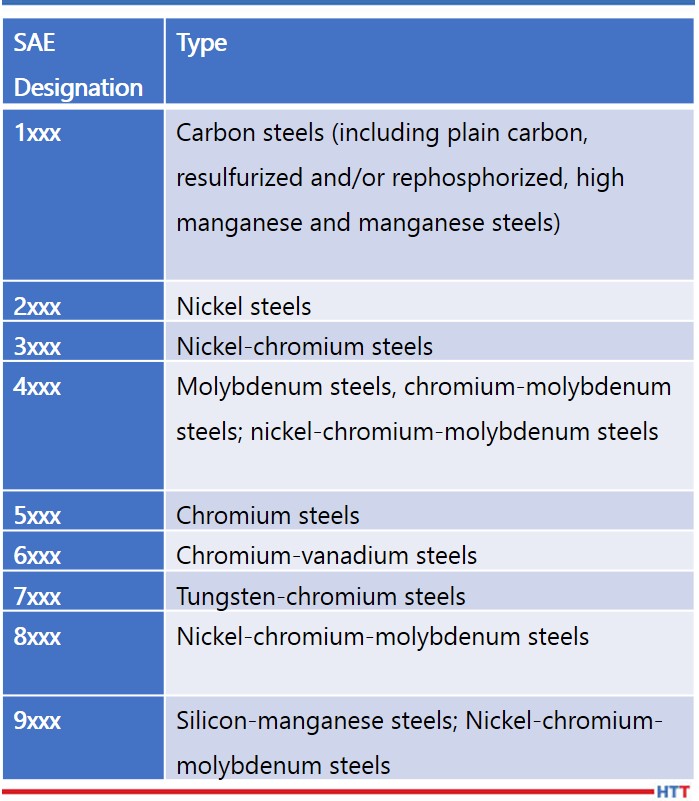
Successful heat treating begins by understanding the make-up of the steel that is to be treated. The American Iron and Steel Institute (A.I.S.I.) and the Society of Automotive Engineers (S.A.E.) utilize a four-digit system to code various types of steel used in manufacturing. The alloying element in the AISI specification is indicated by the first two digits, and the amount of carbon in the material is indicated by the last two digits. The first digit represents a general category of the steel groupings, meaning that 1xxx groups within the SAE-AISI system represent carbon steel. The second digit represents the presence of major elements which may affect the properties of steel; for example, in 1018 steel the zero in the 10xx series depicts no major secondary element. The last two digits indicate the percentage of carbon concentration. SAE 1018 indicates non-modified carbon steel containing 0.18% of carbon, while SAE 5130 indicates a chromium alloy steel containing 1% chromium and 0.30% carbon.
Carbon steel has a main alloying constituent of carbon in the range of 0.12% to 2.0%. Plain carbon steel is usually iron with less than 1% carbon, plus small amounts of manganese, phosphorous, sulfur and silicon. Carbon steel is broken down into four classes based on carbon content:
- Low Carbon Steel: up to 0.3% carbon content
- Medium Carbon Steel: 0.3 – 0.6% carbon content
- High Carbon Steel: 0.6 – 1.0% carbon content
- Ultra-High Carbon Steel: 1.25 – 2.0% carbon content
The Austempering and Quenching Process
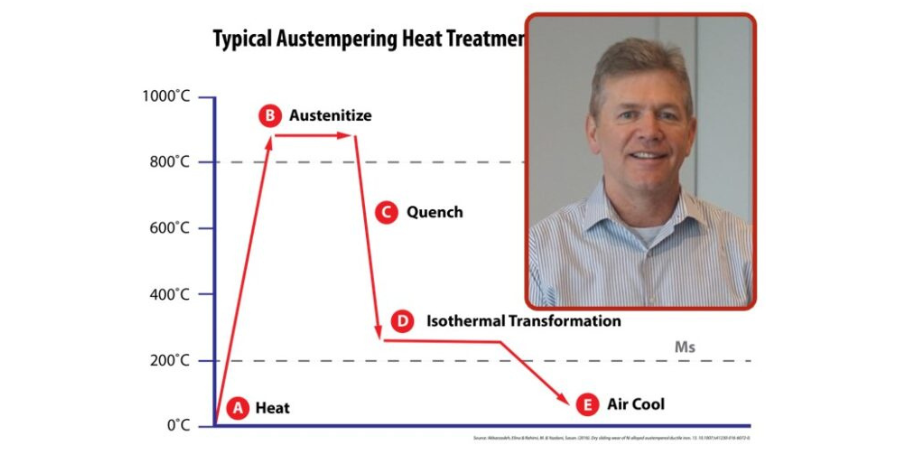
Austempering is one of several heat treatments that is applied to ferrous metals and is defined by both the process and the resultant microstructure of the work. In steel, it produces a bainite (or a plate-like) microstructure.
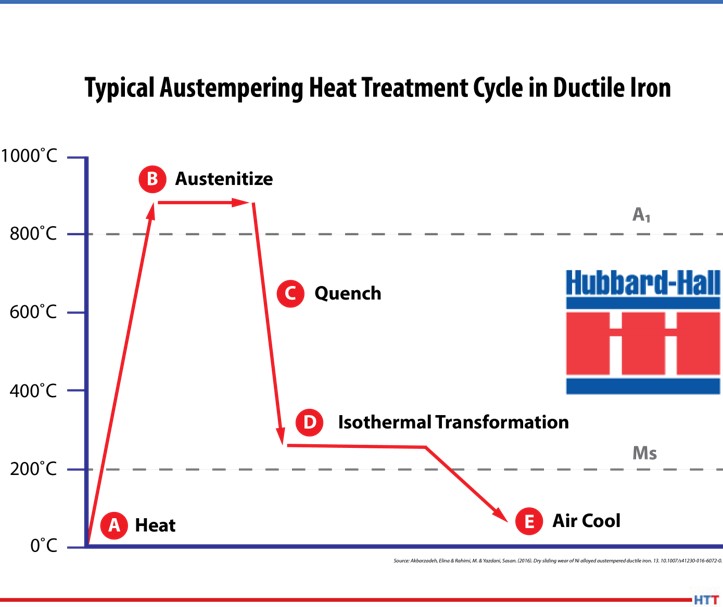
When heated to temperatures below 730°C (1346°F), the pure metal iron has a body-centered cubic structure; if heated above this temperature, the structure will change to a face-centered cubic. On cooling, the change is reversed, and a body-centered cubic structure is once more formed. The importance of this reversible transformation lies in the fact that up to 2.0% carbon can dissolve in a face-centered cubic, forming what is known as a “solid solution.” While in a body-centered cubic iron state, no more than 0.02% carbon can be dissolved this way. The solid solution formed when the carbon atoms are absorbed into the face-centered cubic structure of iron is called austenite.
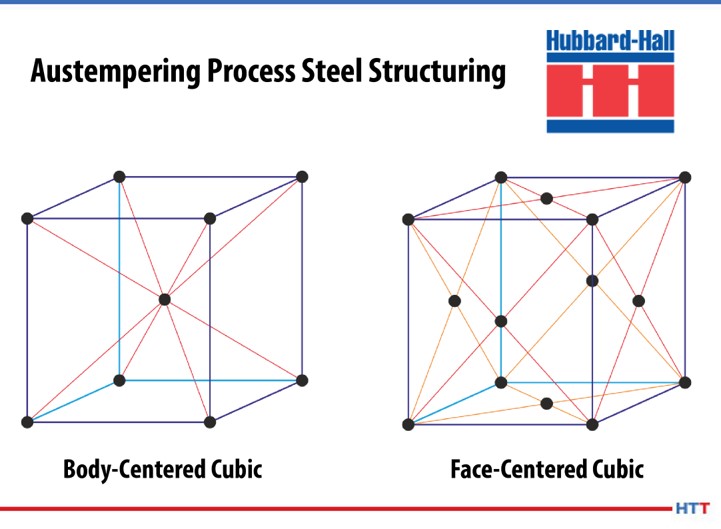
When quenched, carbon is precipitated from austenite not in the form of elemental carbon (graphite), but as the compound iron carbide Fe3C, or cementite. Like most other metallic carbides, this substance is usually very hard; as the amount of carbon increases, the hardness of the cooled steel will also increase.
The temperature of the quench tank is set so that the material is rapidly cooled down at a rate fast enough to avoid transformation to intermediate phases such as ferrite or pearlite and then held at a temperature that falls within the bainite region but staying above the martensitic phase. The bainitic microstructure that is formed as a result of austempering imparts high ductility, impact strength, and wear resistance for a given hardness; a rifle bolt was one of the first applications for this process.
The salt quench also provides low distortion of work with repeatable dimensional response. The materials have increased fatigue strength and is, in general, more resistant to hydrogen and environmental embrittlement.
Heat Treat with Salt Baths
Salt bath heat treatment is a heat treatment process comprising an immersion of the treated part into a molten salt, or salts mixture.2 There are numerous benefits of heat treatment in salt baths, the most prevalent is that they provide faster heating. A work part immersed into a molten salt is heated by heat transferred by conduction (combined with convection) through the liquid media (salt bath).2 The heat transfer rate in a liquid media is much greater than that in other heating mechanisms, such as radiation or convection through a gas.2
Using salt baths also helps with a controlled cooling conditions during quenching. In conventional quenching operation, typically either water or oil are used as the quenching media and the high cooling rate provided by water/oil may cause cracks and distortion. Cooling in molten salt is slower and stops at lower temperature and avoids may of the pitfalls associated with a faster quench.2
Salt baths also provide low surface oxidation and decarburization, as the contact of the hot work part with the atmosphere is minimized when the part is treated in the salt bath.2 There are additional advantages to salt heat treat:
- Wide operating temperatures: 300°F -2350°F
- Most of the heat is extracted during quenching by convection at a uniform rate.
- Salt gives buoyancy to the work being processed to hold work distortion to a minimum.
- Quench severity can be controlled or manipulated by a greater degree by varying temperature, agitation and water content of the salt.
- Excellent thermal and chemical stability of the salt means that the only replenishment required is due to drag-out losses.
- Nonflammable salt poses no fire hazard.
- Salt is easily removed with water after quenching.
References:
- “What is Heat Treating?” ASM International. https://www.asminternational.org/web/hts/about/what-is
- Dmitri Kopeliovich, “Salt Bath Heat Treatment,” SubsTech. https://www.substech.com/dokuwiki/doku.php?id=salt_bath_heat_treatment
- AISI/SAE Steel and Alloy Designation System, The Engineering Toolbox. www.engineeringtoolbox.com
About the Author: Jerry Dwyer is Hubbard-Hall’s market manager for product groups pertaining to heat treating, phosphates and black oxide. To learn more or get in touch, please visit Hubbard-Hall’s website.