In this installment of Answers in the Atmosphere, David (Dave) Wolff, an independent expert focusing on industrial atmospheres for heat treat applications, explores the versatile role of nitrogen gas in thermal processing.
This informative piece on nitrogen’s critical functions in safety, as a diluent, and as an atmosphere component — including production methods and purity requirements — was first released in Heat Treat Today’s November 2025 Annual Vacuum Heat Treating print edition.
As discussed in the introduction for this series of gas-focused columns, nitrogen gas is ubiquitous in thermal processing — by far the most-used delivered or generated gas in secondary metallurgy. This column covers many important considerations for the use and availability of nitrogen gas, featuring the insights from my recent interview with Air Products experts: John Dwyer, principal engineer; Bryan Hernandez, commercial technology sales engineer; and Emily Phipps, strategic marketing manager. Because of its key role in thermal processing, we expect to have additional columns on nitrogen gas in this series.
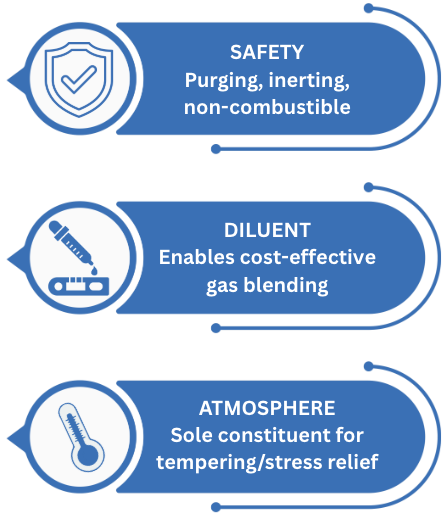
Nitrogen serves three important purposes in secondary metallurgy:
- Safety
- Diluent
- Atmosphere
Regarding safety, the Air Products experts shared important attributes of nitrogen and several applications it is most often used in. According to them, nitrogen:
- will not react with most metals used in fabrication applications until reaching extremely high temperatures
- will not support combustion or oxidation
- has about the same density as air (which is 78% nitrogen)
- is the least expensive industrial gas on a volumetric basis.
For those reasons, nitrogen is used as a purging and inerting gas in metallurgical applications, such as inerting the furnace in preparation for a flammable atmosphere to be introduced, as well as expelling flammable atmosphere at the end of a furnace cycle. They further noted that the National Fire Protection Association (NFPA) Standard 86 for Ovens and Furnaces mandates that nitrogen be always available for furnace inerting except for very specific exceptions where alternative approaches are used (burn in and burn out). Beyond the strict safety considerations, nitrogen protects furnace linings and components from high temperature oxidation.
Dwyer, Hernandez, and Phipps emphasized that when used as a diluent, nitrogen makes it possible to use relatively small volumes of a more expensive reactive gas or gas blend and ensure that the diluted active gas can provide benefits for an entire furnace load of parts. Examples include nitrogen/hydrogen atmospheres where nitrogen gas can enable a relatively small volume of very powerful reducing gas hydrogen to be mixed with a higher volume of nitrogen to fill the furnace interior. I would add that a blended atmosphere of nitrogen/hydrogen will have a higher density than hydrogen alone, and hence may distribute more widely in the furnace rather than just pooling at the ceiling level.
They further discussed how nitrogen can be used as a sole constituent in a furnace atmosphere in many cases, especially at lower temperature ranges, such as tempering and stress relief. In situations where surface finish is a secondary consideration, or where additional operations are going to be performed, they note that the part lower finish quality provided under inert nitrogen alone might be acceptable.
The team then reported that nitrogen forms the bulk of the atmosphere and cryogenic air separation is now available virtually worldwide; because of this, liquified or gaseous compressed nitrogen can also be delivered to clients virtually worldwide. Cryogenically separated nitrogen is, by the nature of the process, extremely pure, and can be assumed to be 99.999% or purer as delivered into the client’s storage vessel. Nitrogen can also be made at the client’s site, using non-cryogenic or cryogenic air separation techniques. For secondary metallurgy, non-cryogenic techniques are the most common because the volumes of nitrogen required are too low for a dedicated cryogenic air separation unit.
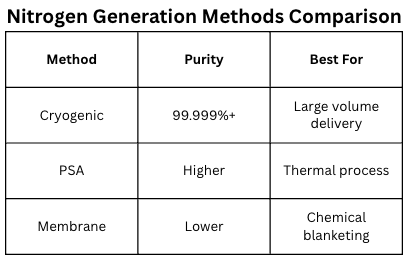
Continuing along this line, they explained that while both pressure swing adsorption (PSA) and hollow fiber membrane techniques can be employed to generate nitrogen for a single customer site, the PSA technology is the one primarily used to supply generated nitrogen for thermal processes. This is because the membrane technique for non-cryogenic nitrogen generation makes relatively impure nitrogen, with too much oxygen to achieve the desired surface properties sought by heat treaters. As such, membrane generated nitrogen is primarily used for chemical blanketing and similar low temperature air displacement applications.
The final discussion point I will share from the interview today is about the variability in accepted purity based on the planned usage of nitrogen. The three Air Products experts pointed out that NFPA86 mandates that the atmosphere in a furnace must be below 1.0% oxygen before any flammable gas species can be introduced. So, they continued, nitrogen used solely for safety purging can be relatively impure and still achieve the 1.0% maximum oxygen allowed. When used as the sole atmosphere component (i.e., 100% N₂), or as a carrier gas blended with an active gas like hydrogen, they explained that nitrogen purity must be much higher in order to achieve acceptable surface quality. In general, for atmosphere uses, it should be assumed as a general rule that the purer the nitrogen is, the easier it is to achieve satisfactory heat treat results. The three concluded this thought noting that in blended atmospheres it may be possible to use slightly higher levels of active gases (like hydrogen) to react with excess oxygen in the nitrogen supply, but that approach is unlikely to make sense economically since nitrogen is typically far less expensive than an active gas.

In the December 2025 installment of Answers in the Atmosphere, I’ll share further insights that my interview uncovered. Until then, consider your unique nitrogen needs and therefore whether having direct access to this gas for the benefit of your heat treat operations is essential.
About The Author:

Independent expert focusing on industrial atmospheres for heat treat applications
Dave Wolff has over 40 years of project engineering, industrial gas generation and application engineering, marketing, and sales experience. Dave holds a degree in engineering science from Dartmouth College. Currently, he consults in the areas of industrial gas and chemical new product development and commercial introduction, as well as market development and selling practices.
For more information: Contact Dave Wolff at Wolff-eng@icloud.com.