Heat Treat Radio host, Doug Glenn, and several other Heat Treat Today team members sit down with long-time industry expert Dan Herring, The Heat Treat Doctor®, to talk about simplified mill practices and processes as they relate to aluminum and steel. Enjoy this second informative Lunch & Learn with the Heat Treat Today team.
Below, you can watch the video, listen to the podcast by clicking on the audio play button, or read an edited transcript.
The following transcript has been edited for your reading enjoyment.
Dan Herring (DH): It’s my pleasure to be here and what I’m going to attempt to do in about the next 30-40 minutes is take about 3 or 4,000 pages of literature and condense it down into some simple English and some common sense, if you will.
We will talk about mill practices, production methods, and what I like to call the forms produced. We might call this whole thing “simplified” for lack of a better terminology, if that makes sense. I’ve selected two very common materials to talk about. The first one is aluminum and the second is steel. But I’m going to disguise that a little bit and talk a little about aluminum and iron. Just to recall, maybe our high school chemistry, aluminum (or aluminium as it’s called by the rest of the world), has chemical symbol Al and iron has chemical symbol Fe. You might wonder how we got Fe from iron: it’s from the Latin word ferrum. Aluminium is another story which I’ll leave for another time, but it is quite interesting.
If we’re going to talk about aluminum and if we’re going to talk about iron, why isn’t steel an element? That’s a question I get very often. Steel is actually an alloy. That’s a combination of different elements. The way I like to think about steel is it’s iron and manganese and carbon and some other alloying elements put in that make specific types of steel that are used for specific applications and application purposes.

The other common question I get is you’ve heard of terms in history like “the stone age” where all the tools and, by the way, the weapons were made of stone. Similarly, the stone age gave way to something called “the bronze age.” That’s where an alloy of copper and tin came on. Again, it made better tools and, by the way, better weapons than the stone tools were. Then, later, you probably heard that there was something called “the iron age”, and we all commonly have heard these terms, but why haven’t we heard about “the steel age”? That’s a common question. What is the steel age? Why isn’t it an age, if you will? That’s because we came up with a very fancy term: The Industrial Revolution, where we started to use steel as an engineering material. I don’t want to get too off subject here, but thought I’d mention that.
So, we begin with raw material, and we call that within the industry an ore. Now, most raw material is in the form of ore or minerals that are found in nature, and they’re typically the element of interest (aluminum or iron in this case) combined with possibly some undesirable elements. The ore that we get from the raw material that we get from the earth has to be refined to make it into a metal. And there are certain raw materials (gold is a good example), that are found in its pure state. I which I could have found more of it in my career, then I wouldn’t be talking to you, but that’s a different story! The idea here is the fact that most ores come in the form of, or most minerals are found in nature and have to be refined.
[blockquote author="Dan Herring, The Heat Treat Doctor®" style="1"][The] chemical bond between aluminum and oxygen is very strong. As a result of that, we need a lot of energy to break that bond apart, to produce aluminum the metal and oxygen the byproduct. A lot of energy is required for that[/blockquote]
The principal ore containing aluminum is something we call bauxite. Bauxite is aluminum oxide, chemical symbol Al203. The way I like to think of bauxite is bauxite is dirt. We can put a dress on it, but it’s still dirt at the end of the day. It’s a special type of dirt. It’s a dirt that has 40-60% aluminum oxide in it. And there are certain areas in the world where bauxite is more common than others. Interestingly enough, Australia is a tremendous source of bauxite as is Africa. That’s why you find the majority of bauxite mines in either Australia or Africa or other places in the world.
When you get into iron, there are two principal ores — there are hematite and magnetite. They are iron oxides and they’re obviously rich in iron.
But to begin, let’s deal with aluminum and what the mill has to do, or what the aluminum manufacturing process really is. We start off, as I said, with dirt, with the raw ore. We then get fancy, and we crush it into a very coarse powder and then after we’ve crushed it, we want to refine it — we want to take and remove some of the impurities. So, we mix it with a little of what we call caustic soda, which is sodium hydroxide, and lime, which is calcium oxide or calcium carbonate, and we use that refining method to purify the raw ore. What we wind up with, interestingly enough, is a very fine white powder which is called alumina or aluminum oxide.
We start out the manufacturing process with a raw material that is a very, very fine powder that is almost all (principally 99%) aluminum oxide. We take it and we put it into a furnace, and we heat it. We do that process with electricity because we’re using carbon anodes, if you will, placed into the bath that we pass current through to melt the aluminum. The process therefore is extremely energy intensive. That’s why you find aluminum production plants in areas like the Tennessee valley, where we have a lot of hydroelectric power. You find them in Iceland, where you have a lot of geothermal energy to help produce electricity. But they’re very electrically intensive operations.
The scientific reason for that is that the chemical bond between aluminum and oxygen is very strong. As a result of that, we need a lot of energy to break that bond apart, to produce aluminum the metal and oxygen the byproduct. A lot of energy is required for that.
You might also find it interesting that when the process was first developed back in the 1880s, and it took that long to produce pure aluminum — if I remember right, the year was 1883 — but the price of an ounce of aluminum was more expensive than the price of an ounce of gold just because of the manufacturing of it.
But anyway, we’ve taken this aluminum powder, which is a white powder, we’ve melted it into a silvery-colored metal, and we do that inside a furnace. Then we tap the furnace — in other words, we pour out the molten aluminum and we either produce cast products from the aluminum or we produce what are called ingots for subsequent working. We either make castings directly or we make ingots.
Cast products, examples of them, might be engine blocks, wheel rims for automobiles, even some small appliances (there are toasters that are cast), patio furniture, tools, cookware — a lot of things wind up just as cast products.
But if we’ve produced an ingot, now we have various methods that we take to produce an engineered product, if you will. We can extrude the aluminum — in other words, we can take an aluminum ingot and we can put it in a press and press it into a form and we can make things like aluminum ladders, bicycle frames, even certain airframe components, out of extruded material. We can take these ingots and we can roll them — we can roll them hot, or we can roll them cold — this is called hot rolling and cold rolling.
But we can turn around and when we roll it, we can make sheet, we can make plate, we can make something that we’re all very familiar with which is aluminum foil. We can make wire, heat exchangers, panels for automobiles, and battery components. Again, in the transportation industry, we can make a lot of things for automobiles or airplanes.
Similarly, we can also forge the material. We hot forge it in this particular case, but we can make various rings and blocks and cylinders and sleeves and components that we can then take and machine.
The process of manufacturing aluminum is relatively straightforward, and it winds up, as I said, with an ingot of some type that is then manufactured into a product.
Doug Glenn (DG): I want to jump in with two thoughts:
You’re talking about that the manufacturing of aluminum from raw materials is highly energy intense. Two points on that: One, it’s much more energy intense than steel production, for one thing, and secondly, that makes some sense of why it is we do so much recycling (or at least try to) of aluminum, because it’s a lot cheaper to take already formed aluminum (an aluminum can or an aluminum wheel off a car) and melt it down. The amount of energy to do that is a lot less than it is to create aluminum from scratch. That was one thing, Dan, if you want to comment on that.
The second thing is you were talking about extruding. I imagine that most everyone knows what that is. You were talking about pressing it into a form. You’ve got to remember that with an extrusion, you’re pressing it through a dye. It’s kind of like your playdough that you push in that form, and you get a shape coming out the other end — that’s extrusion, and not to be confused with forging where you’re putting it into a closed thing and pressing it into a form.
DH: Those are both very, very good comments. Interestingly enough, when you get into iron and steel making, the minerals, the iron oxides if you will, are far easier to break the bond between iron and oxygen than it is between aluminum and oxygen. That’s why the aluminum is such an energy intensive process.
And absolutely correct — recycling saves a tremendous amount of cost and is something that is vital to the long-term success of aluminum because an aluminum product, in general, is more expensive than a steel product.
You are correct — when you extrude something, you basically squeeze it through a dye, if you will. We’ll talk about that a little bit more in forging.
I want everyone to understand that when we start to talk about iron and steel making, because the process has been around for such a long time, there are certain terms that are used in the manufacturing process that have become synonymous with the process itself. Once again, we start out with an iron oxide, a mineral in the form of magnetite or hematite. We take that raw ore and we put it into something called a blast furnace. This is where we do a process called “smelting” of the material. We form a metal by taking and reducing the ore in the presence of air under pressure.
Coming out of the blast furnace is molten metal, molten iron, if you will. Now, historically, it’s called “pig iron.” The reason for that is when they originally cast different molds with shapes, the resulting structure looked like a litter of piglets that were actually suckling on their mother. So, the term “pig iron” came about. These little “pigs,” if you will, were broken off from the main casting. As I said, there are a lot of historical things going on.
In the old days, you then took the pig iron and you put it into what is called either a BOF (basic oxygen furnace) or an EAF (electric arc furnace) and then you remelted the pigs, if you will. But today, in most of the BOF and EAF processes, you wind up charging a hot liquid iron into those furnaces. They heated up, or continued to heat up, and then you turn around after you’ve converted the pig iron (which is about 94% iron and 6% impurities, so it’s still very impure) and with processing in a BOF or EAF furnace, you get the impurity levels down to less than 1%.
You might say to yourself, “Why is that important?” The idea in steel making is to take the raw material — the iron — and take everything out of it, so we can precisely add back in just those chemical elements that we want to make a particular type of steel. That’s essentially what the BOF or EOF is doing it; it’s converting the molten metal (or the pig iron) into a very, very pure material.
We then do a process which is called “tapping.” We transfer the raw material into a ladle furnace and inside the ladle is where we do the remainder of the refining process. What we wind up doing is we purify the material — we get rid of the additional impurities that are present, anything from hydrogen and oxygen and excess nitrogen to tramp elements and things of this nature. So, in the ladle, we do the refining. This can be done in a vacuum process, a vacuum degassing process, it can be done with an argon process, if you will. But we go from the blast furnace to the refining furnace (the BOF or the EAF), we then go into the ladle and what we’re doing is we’re taking the raw material and we’re making a purer and purer and purer form of, first of all, iron, and then we’re starting to add in elements that we want to make a particular grade of steel or type of steel. Then we’re going to do a process called “teeming” and “casting.” Teeming is basically pouring the molten metal into molds.
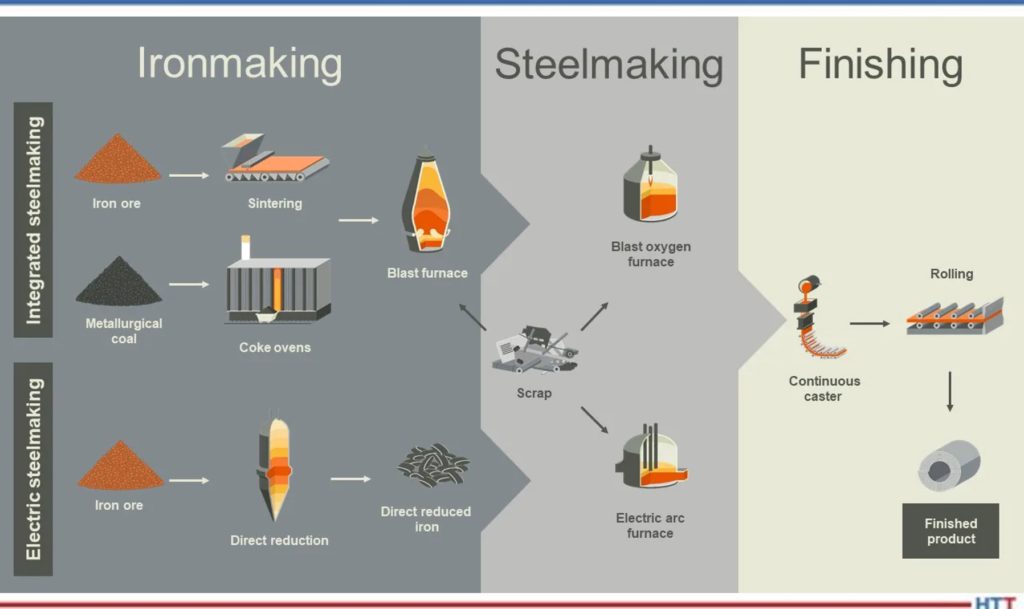
What we wind up with is we have a process where we have liquid steel and we’re going to send it into either something called a continuous caster, we’re going to make ingots out of it, or we’re going to take and atomize the steel. I want to talk about atomizing the liquid steel first. The process is done by adding a gas such as nitrogen or argon or even air, or by using water, but the idea here is that what you wind up with is a powder metal.
By the way, it’s called “powder” metallurgy not “powdered” metallurgy. Powdered is cookies, but powder is what we produce from the atomizing process. The powder can either be spherical in nature or it can be rounded or even irregular-shaped, depending on the type of atomization process. But we take this liquid stream of metal, and we impinge it with either water or gas and burst it or break it apart into particles. Then we do a simple process which is called screening of those particles — it’s basically taking and getting finer and finer, or dividing the powder into finer and finer powders.
Depending on the purification of the powder, how fine the powder is, we use it for what we call conventional powder metallurgy, so we take and use it for basic sintering operations, for example. You’re all familiar with the rearview mirror on your automobile. Interestingly enough, the rearview mirror fits into something called a mirror mount, and that mirror mount is a powder metal part. It happens to be a stainless steel, but it’s a powder metal part.

The idea is the fact that we can have a conventional powder metal. We can have (if we use finer powder) a metal that is suitable for metal injection molding for making things like firearm components, orthodontic braces and things of this nature, or other medical-type devices. Or, if we get a superfine powder, we can turn around and we can use it for something called additive manufacturing.
We’ll talk a little bit more about these later, but from the casting process, we can either go into a continuous caster, we can make ingots, or we can atomize the liquid steel.
If we go into a continuous caster, we’re cooling down the steel and we’re producing three products — they’re called blooms, billets, and bars. Basically, the difference between them is their physical shape. A billet might only be 10 inches square or something of this size (10 x 10 x 10 inches). A bloom is defined as something that is less than one hundred square inches, typically, except if it’s a jumbo bloom caster which makes bigger blooms, but we’ll ignore that as it gets complicated quickly.
The idea here is the fact that we’re either going to take the liquid steel, we’re going to cool it down in some continuous fashion or we’re going to put it into a mold to make an ingot or we’re going to atomize it using water or a gas to make a powder. Those are the three forms that come out of this whole process.
DG: Dan, I’ve got a quick question for you on that: With the aluminum, you mentioned that you can melt it and then cast it directly into a finished product (a cast product). Do we do that much with steel? Do we often take steel and actually take it directly into an alternator casing or some other finished part?
DH: Absolutely. There is a lot of cast steel that is used. The example that comes quickly to mind are probably valve bodies that are used in the petrochemical industry and things. If you think about the iron side, you’re very familiar with cast iron skillets and cast iron cookware. You can also have steel castings as cookware, but you typically don’t as it’s more expensive. But yes, you can make a variety of products directly as a casting.
As I said, you can make powder metallurgy products, and you can also make a family of products that we then call wrought products. What we do is we take those billets, blooms, and bars and then we either hot work them or cold work them to make various types of materials. We can roll them, we can pierce them, we can forge them. We can make sheet, we can make plate, we can make bar and tubular products, we can make wire, we can make strip. A good example is the fact that if you’re a razor blade manufacturer, you want to order material from the mill that’s in the form of strip, thin strip actually.
If, on the other hand, you’re in the oil and gas industry, and if you’re ordering pipe or tubing for use, as we call it, “down hole”, obviously it does no good to have delivered a strip of steel or a sheet of steel or a plate of steel, you want something obviously in the form a tube or a pipe that can then be used.
For more information:
dherring@heat-treat-doctor.com
To find other Heat Treat Radio episodes, go to www.heattreattoday.com/radio and look in the list of Heat Treat Radio episodes listed.
 Find heat treating products and services when you search on Heat Treat Buyers Guide.com
Find heat treating products and services when you search on Heat Treat Buyers Guide.com