In the following original content from Heat Treat Today, Thermo-Calc Software's Adam Hope, PhD, materials scientist, and Paul Mason, president, delve into how modeling and simulation tools can help heat treaters make well-informed decisions.
In the following original content from Heat Treat Today, Thermo-Calc Software's Adam Hope, PhD, materials scientist, and Paul Mason, president, delve into how modeling and simulation tools can help heat treaters make well-informed decisions.
This article first appeared in the latest edition (March 2020) of Heat Treat Today’s Aerospace Heat Treating magazine.
Consistency in material properties and performance is critical to the aerospace industry, and small variations in material chemistry or process windows can have a large impact on the final parts performance. The ability to predict and adjust for these variations can reduce scrap and part re-work. Metallurgists and process engineers responsible for heat treatments must adapt their process when input variables change, such as material chemistry. They are routinely faced with questions such as:

President
Thermo-Calc Software
*How will heat to heat variations affect the final part performance?
*What heat treatment should be given to a part that has been built via a novel approach such as additive manufacturing?
*How should one optimize a heat treatment schedule for a new alloy?
*When the data required to make these decisions does not exist, what are the options?
Experiments can generate this data, but this is costly and time-consuming. Handbooks might have data for known alloys, but this is often only for the nominal composition and may not be suitable for material processed under a novel route. Modeling and simulation tools can help fill this knowledge gap and help inform better decisions.
Integrated Computational Materials Engineering and CALPHAD

Materials Scientist
Thermo-Calc Software
The publication by the National Academies in 2008 on Integrated Computational Materials Engineering (ICME)[1] outlined an approach to designing products, the materials they are comprised of, and their associated materials processing methods, by linking materials models at multiple length scales. The report highlighted the need for a better understanding of how processes produce material structures, how those structures give rise to material properties, and how to select materials for a given application, describing the need for using multiscale materials modeling to capture the process, structures, properties, and performance of a material.
Computational thermodynamics, and specifically CALPHAD (CALculation of PHase Diagrams)[2], enables the prediction of the thermodynamic properties and phase stability of an alloy under stable and metastable conditions. The CALPHAD approach captures the underlying composition and temperature dependence of properties and can also be extended to model atomic mobilities and diffusivities in a similar way. By combining thermodynamic and mobility data, kinetic reactions during solidification and subsequent heat treatment processes can be simulated. Computational thermodynamics and CALPHAD- based tools are an important component of an ICME framework because, through the use of such simulations, it is possible to vary alloy compositions and predict optimal solidification processes and solution heat treatment temperature ranges without performing many time-consuming and costly experiments.
Predicting Heat Treatments for Additively Manufactured Parts
Many additive manufacturing processes subject the material to rapid solidification with multiple subsequent reheat cycles. The effect of these thermal cycles on material properties is not always known and typically does not result in the properties that a similar cast or wrought metal would have. Additionally, many additively manufactured parts are built using conventional alloys which have been engineered for cast or wrought processes. In some cases these alloys are not suitable for additive processing, and problems such as deleterious phases forming during a post-build, stress-relief heat treatment, designed for conventionally treated alloys, may result.
Additive processes are typically associated with rapid cooling rates and large thermal gradients. This can give rise to the following:
- High levels of residual stress in the final part
- Microsegregation during solidification of each layer, which leads to local inhomogeneities in alloy composition
In the case of additive manufacturing, these separate heat treatments are often combined, and stress relief heat treatments designed for cast or wrought material may not be suitable for additively processed materials for two reasons:
- The chemical inhomogeneities arising from rapid cooling can influence precipitation behavior, and some deleterious precipitates may precipitate more quickly than expected.
- The multiple heating cycles of subsequent layers may have already started some precipitation reactions, making stress relief more difficult without first homogenizing these precipitates.
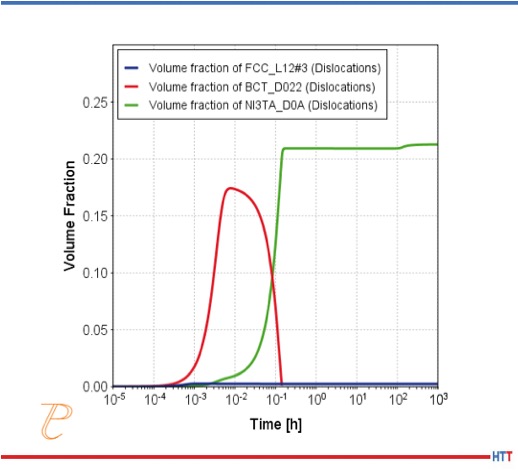
Zhang et al.[3] have studied laser powder bed builds of Alloy 625 and found that after applying an industry recommended stress relief heat treatment, delta phase can precipitate in the segregated regions much faster than in the wrought material. The formation of delta phase is extremely detrimental to material properties. They attributed this to increased Nb and Mo concentrations found in the interdendritic regions in the as-built microstructure.
To understand this further, the authors first simulated the extent of this segregation using the Scheil-Gulliver model for solidification in Thermo-Calc[4] in conjunction with the diffusion module, DICTRA[4]. They then used the Precipitation module, TC-PRISMA to predict the precipitation kinetics of the deleterious delta phase for nominal feedstock compositions, as well as the compositions measured at dendrite boundaries. Both simulations, shown in Figures 1a and 1b, predict that a stable MC carbide forms, followed by some gamma double prime. Delta phase then forms at the expense of the gamma double prime. However the gamma double prime and delta phase both precipitate much more quickly in the segregated interdendritic region, due to the increased Nb and Mo. Delta phase is predicted to start forming around 1 hour, compared with 10 hours for the wrought material.

While these calculations give insight to the reason why the conventional stress-relief heat treatment is not suitable, additional simulations can be made to identify a suitable temperature and time to both homogenize and stress-relieve the part, while avoiding deleterious phases. The authors of the study determined a post-build homogenization treatment was required to avoid deleterious delta phase precipitation.
Gas Carburizing Highly-Alloyed Steels
Highly-alloyed stainless steels can be gas carburized to increase the surface hardness, as well as improve the overall mechanical characteristics of the surface. However, an increase in chromium-rich carbides such as M23C6 or M7C3 can result in the decrease of chromium in the solid solution which leads to a reduction in corrosion resistance. Balancing these properties can be time consuming through trial and error experimentation, but CALPHAD-based tools can be used to identify suitable alloy compositions and heat treat windows, which are optimal for the application needs prior to testing in the laboratory.
Turpin et al. 5 made such a study, combining both experimental work and theoretical simulations to investigate carbon diffusion and phase transformations during gas carburization of high alloyed martensitic stainless steels. First, using thermodynamic calculations performed with Thermo-Calc 4 they determined the optimal balance between the carbide formation and chromium content of the alloy for corrosion resistance. They concluded:
- At 1750°F (955°C), which corresponds to the austenitization temperature of their alloy, M23C6 and then M7C3 will be the first carbides to precipitate in the austenite phase as the amount of carbon content in the alloy is increased.
- If the amount of carbon exceeds 3.8 wt% then M3C carbides are predicted to be stable. M3C carbides have a structure similar to cementite and preferentially precipitate at the grain boundaries which weaken the microstructure. Therefore, to avoid these phases, the overall content of carbon in the steel must be below this amount at the end of the carburizing process.
- Above 1.7 wt% C, the mole fraction (an indicator of the volume fraction) of M7C3 carbides exceeds 20%, and the chromium content of the alloy associated with these carbides is 65 wt%. Therefore, there is a correspondingly strong depletion of chromium from the matrix.
- To balance the desire for adding carbon into the matrix phase to obtain hardness with depleting the matrix of carbon, it was determined that the optimal amount of carbon in the matrix phase should not exceed 1 wt%. Thus, the thermodynamic calculations were used to establish a limit, without yet any consideration of the kinetics or time.
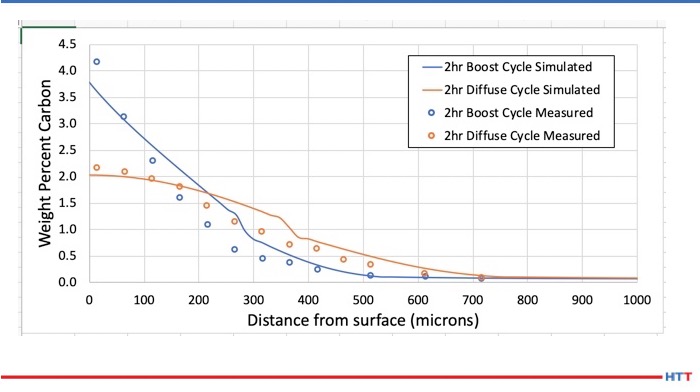
The second stage of the study then considered the diffusional reactions in the multi-component system during i) the carbon enrichment step and ii) the diffusion step of the gas carburizing process, and to determine how the composition and the amount of each phase vary with time and distance from the gas/solid interface and the carbon profile of the alloy as a function of time and distance. These calculations were made using the diffusion simulation software, DICTRA 4.
Consider the carbon enrichment step first. In DICTRA, several boundary conditions can be used for such a simulation, and in this work the carbon flux was determined experimentally using thermogravimetric measurements. During the diffusion step, the N2-CH4 mixture is replaced with pure N2, and the carbon flux at the surface of the samples is zero. To simulate this step using DICTRA, a zero carbon flux was applied as the boundary condition for two hours.
Figure 2 shows a simulated carbon profile for Fe-13Cr-5Co-3Ni-2Mo-0.07C, which is found to be in good agreement with the experimental values reported by Turpin et al. The authors concluded from this study that the carbon profile can be calculated and followed at any time if the boundary condition evolution at the gas-solid interface is known during the carburizing treatment.
Predicting β-transus Temperatures in Ti-Alloys
Many Titanium alloys respond well to heat treatments, through which the microstructure can be manipulated to optimize properties for a particular application. For example, some microstructures are better for high temperature creep, and some are better for fatigue strength. This is primarily achieved by controlling the nature and amount of α and β phases in the microstructure.
At high temperatures, titanium alloys are primarily β phase. At the β-transus temperature, the α phase becomes stable and can start to form. The β-transus temperature can change as a function of alloy chemistry. Knowing the β-transus temperature is critical to determining the nature and amount of α phase that will form during a heat treatment or thermal cycle.
Many alloying elements in Ti alloys have a strong effect on β-transus temperature. Knowing the actual β-transus for a specific chemistry is critical to determining suitable heat treating windows to obtain a specific set of material properties. However, even within a particular alloy specification, there can still be small compositional variations that have a significant effect on the β-transus. Thermo-Calc [4] can be used to calculate this if the exact chemistry is known, or to determine the potential distribution of β-transus temperatures for a given chemistry range.
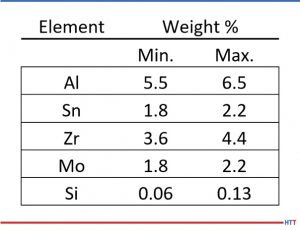
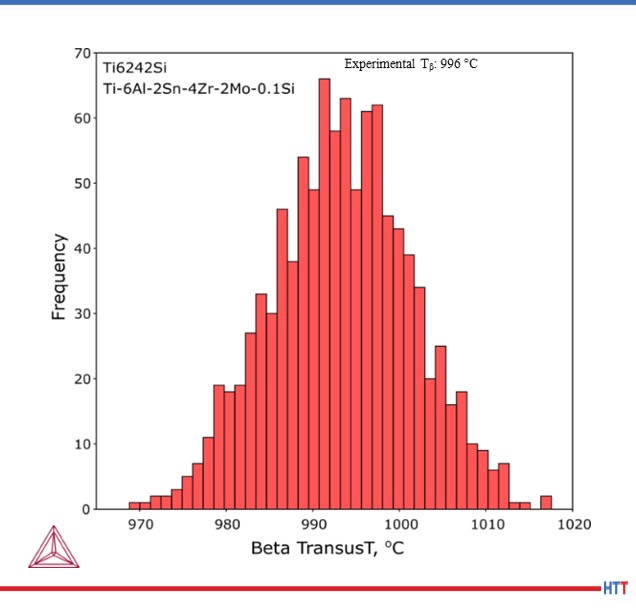
Table 1 shows the composition ranges for the major elements in Ti6-2-4-2Si (AMS 4919). Figure 3 shows the calculated distribution of β-transus temperatures in the composition specification of Ti6-2-4-2. Over 40 degrees variation is possible for compositions that lie within the specification and the calculations show good agreement with the experimentally measured values of 995°C ± 15°C (1823°F ± 27°F).[6]
Summary
In the 100th Column of the Heat Treat Doctor [7], Dan Herring, stated that heat treating can best be defined as “the controlled application of time, temperature and atmosphere to produce a predictable change in the internal structure (i.e. the microstructure) of a material.” However, variability arising from composition differences in materials can sometimes be challenging for heat treaters. The examples shown here have illustrated how modeling and simulation tools such as those based on the CALPHAD approach can be used to predict variability arising due to material composition. HTT
References
[1] National Research Council. 2008. “Integrated Computational Materials Engineering: A Transformational Discipline for Improved Competitiveness and National Security.” Washington, DC: The National Academies Press.
[2] Kaufman, L and Bernstein, H. Computer Calculation of Phase Diagram. New York: Academic Press Inc, 1970.
[3] Zhang, Fan, et al. “Effect of heat treatment on the microstructural evolution of a nickel-based superalloy additive-manufactured by laser powder bed fusion.” Acta Materialia 152 (2018) pp 200-214.
[4] Andersson, J O, et al. “Thermo-Calc and DICTRA, Computational tools for materials science.” Elsevier, CALPHAD, Vol. 26, (2002) pp. 273-312.
[5] Turpin, T, et al. “Carbon diffusion and phase transformations during gas carburizing of high-alloyed stainless steels: experimental study and theoretical modeling.” Met. Trans. A, Vol. 36A, (2005) pp 2751-2760.
[6] TIMET datasheet for TIMETAL® 6-2-4-2, TMC-0157 (2000).
[7] Herring, D “What is Heat Treating and Why Do We Do It?” Industrial Heating Magazine BNP Media (2011).
About the authors: Paul Mason is the president and Adam Hope, PhD is a materials scientist for Thermo-Calc Software, whose products assist academia, government, and industry to make calculations which predict or assist in the understanding of complex multicomponent alloys and non-metallic systems, as well as processes of industrial and scientific relevance.
For more information, contact Paul or Adam at info@thermocalc.com or (724) 731 0074